Atomic Mass, the Mole, and Molar Mass
The Mole
The chemical formula of common salt or sodium chloride is NaCl, indicating that it is a compound formed from the elements sodium and chlorine in a one-to-one atom ratio. The elemental composition of \(58.5\) g of salt is \(23.0\) g of sodium and \(35.5\) g of chlorine. When the reactants, sodium and chlorine, are combined in the mass ratio of 23 to 35.5, sodium to chlorine, there are equal numbers of sodium and chlorine atoms as reflected in the chemical formula. In other words, \(23.0\) g of sodium metal contains the same number of atoms as \(35.5\) g of chlorine gas. Elements combine or recombine with each other as individual particles and in mass ratios that are integer multiples of their relative atomic mass values. Consequently, the mass in grams corresponding to the relative atomic mass value of any element contains the same number of atoms or particles as the mass in grams corresponding to the relative atomic mass value of any other element. Meaning, for example, that \(12.01\) g of carbon, which has a relative atomic mass of 12.01, has as many atoms as \(207.2\) g of lead, which has a relative atomic mass of Pb of 207.2. This also means that two times the relative atomic mass of carbon, or \(24.02\) g of carbon, has the same number of carbon atoms as there are lead atoms in two times the relative atomic mass of lead, or \(214.4\) g of lead.
The relative atomic mass of an element expressed in mass units of grams represents a specific number of individual atoms known as a mole, from Latin meaning mass, and abbreviated as mol. A mole of any element is its relative atomic mass in mass units of grams, and is known as the molar mass. One mole represents a specific number of atoms or particles present in the molar mass of the substance, regardless of the element. This number has an unbelievably large value of \(6.022 \times 10^{23}\) and is known and referred to as Avogadro’s number, in recognition of the Italian scientist Amedeo Avogadro, whose early work helped establish the idea of the mole. Although the mole represents a set number of atoms, the most important point is that it represents equal numbers of atoms based on the relative atomic mass of an element. Each element has its own unique relative atomic mass that equates the amount of a substance measured in mass units with the number of atoms or particles in that mass amount. For example, the relative atomic mass of gold is 196.97, so the molar mass of elemental gold (Au) is \(196.97\) g, meaning that 1 mole Au atoms or \(6.022 \times 10^{23}\) atoms of gold \(=\) \(196.97\) g of Au. Individual atoms are far too small to count, but mass is a convenient way to measure the amount of a material and, consequently, the number of atoms.
Atomic Mass and Moles Transcript
Atomic Mass/Mole Calculation Practice
Molar Mass of Compounds and Molecular Mass
Some elements exist as particles with two or more atoms bound together, forming a molecule. The elemental chemical formulas of two such elements of oxygen and fluorine, are O\(_2\) and F\(_2\). The individual particles of both of these elements exist as diatomic molecules, di meaning two, because the individual molecules are formed from two atoms. The molecular molar mass of these elements is the atomic molar mass of the element times the number of atoms in each particle, or two. The molar mass of diatomic elemental oxygen is \(32.0\) g or \(2 \times 16.0\) ,g and the molar mass of diatomic fluorine is \(38.0\) g or \(2 \times 19.0\) g. The molar masses of diatomic elemental oxygen and diatomic elemental fluorine contain the same number of individual molecules.
The molar mass of any pure compound is the sum of all the relative elemental atomic molar masses present in the compound. If more than one atom of the same type of element is present, the relative atomic mass of that element is multiplied by the number of atoms in the compound. For example, the chemical formula of aluminum oxide is Al\(_2\)O\(_3\). So, the molar mass of aluminum oxide is two times the relative atomic mass of aluminum or \(2 \times 26.98\) g, which is \(53.96\) g, plus three times the relative atomic mass of oxygen atoms or \(3 \times 16.00\) g, which is \(48.00\) g. The molar mass of Al\(_2\)O\(_3\) is \(53.96\) plus \(48.00\) g or \(101.96\) g. Likewise, for compounds that are comprised of individual molecules, the molar mass is calculated from the molecular formula. The molar mass of a molecular compound is referred to as the molecular mass of the compound.
Atoms, Molecules, and Formula Units Transcript
Fundamental Knowledge and Skills - Molar Mass
How to Recognize When You Need to Do It:
When you need to express the mass amount of a substance as a particle amount in units of moles or numbers of individual atoms, molecules, or formula units, or I in the opposite direction, expressing a particle amount in moles or in terms of numbers of individual particles as a mass amount.
How to Do It:
Using the periodic table or another source, add up the atomic mass in grams for each individual element in the material, multiplied by its numerical subscript in the chemical formula. Each element on the periodic table has an atomic mass associated with it. Atomic weights have two possible units. Atomic mass units (amu) are equivalent to roughly the weight of one proton or one neutron and represent the mass of individual atoms. The other unit is grams per mole (mol) and represents molar mass.
The atomic mass of each element can be read directly from the periodic table. If you wish to know the molar mass of the compound ethanol, which has this formula, C\(_2\)H\(_6\)O you need to add up all the atomic masses of the individual elements in the molecule, multiplying each by its subscript. There are two carbons, six hydrogens, and one oxygen. To determine the molar mass of the molecule, follow the procedure below:
Carbon | \(2 \times 12.011\) g\(/\)mol | \(24.022\) g\(/\)mol |
Hydrogen | \(6 \times 1.008\) g\(/\)mol | \(6.048\) g\(/\)mol |
Oxygen | \(1 \times 15.999\) g\(/\)mol | \(15.999\) g\(/\)mol |
Total molar mass of the ethanol molecule \(= 46.069\) g\(/\)mol. Therefore, if you have \(46.069\) grams of ethanol, you have one mole of ethanol molecules, or \(6.022 \times 1023\) ethanol molecules.
Why:
The molar mass of any material in grams has the same number of particles, which is Avogadro’s number or \(6.022 \times 1023\). You will need to convert the mass of a material to moles often as you approach chemistry problems.
Fundamental Knowledge and Skills - Moles/Number of Particles Conversions
How to Recognize When You Need to Do It:
If a question starts with moles and asks for the number of molecules or vice versa, you will need to use this conversion factor.
How to Do It:
Avogadro’s number is \(6.022 \times 10^{23}\). This number represents the number of atoms there are in one mole, regardless of substance. There are \(6.022 \times 10^{23}\) atoms in one mole of atoms, and one mole of atoms has \(6.022 \times 10^{23}\) atoms. This is a conversion factor that you will use to convert from the number of atoms or molecules to moles and back again. To convert from atoms to moles, simply divide by \(6.022 \times 10^{23}\). To convert from moles to atoms, simply multiply by \(6.022 \times 10^{23}\).
\(1\) mol of atoms or molecules \(= 6.022 \times 10^{23}\) particles, atoms or molecules
\(1\) gallon of water contains \(1.265 \times 10^{26}\) molecules of water. This is \(126.5\) septillion molecules of water, more than we can even come close to imagining. Let’s use moles to make this vast quantity of water easier to picture.
\(210\) is a much easier number to picture and use. And this makes sense, as 1 mole of water weighs 18 grams. So, 210 moles weigh 3780 grams, or 3.78 kilograms, which is about 8 pounds, the weight of a gallon of water.
Why:
To modify a quote from Hitchhiker’s Guide to the Galaxy: “Atoms are tiny. You just won’t believe how unbelievably, how mind-bogglingly, incredibly tiny they are. You might think a grain of rice is tiny, but that’s peanuts to atoms.” Atoms and molecules are indeed too small to visualize reactions happening on their scale with any degree of ease. Therefore, we do chemical reactions on a much larger scale so that we can actually observe changes when they occur.
It turns out that Avogadro’s number or \(6.022 \times 10^{23}\) represents the number of atoms that there are in one mole. This is also the same number of hydrogen atoms it takes to weigh one gram. We use Avogadro’s number because of the connection between Avogadro’s number and mass. 1 mole of any given element has the mass, in grams, equivalent to the atomic/molar mass on the periodic table.
Think of Avogadro’s number as a concept similar to a dozen. If there are a dozen donuts, there are 12 of them. If there are a dozen donuts, each with three sprinkles on top of them, there are 12 sprinkled donuts with 3 dozen sprinkles in total. A mole is just a larger dozen. If you had a mole of donuts, assuming each one weighed 40 grams, that pile would weigh \(2.4 \times 10^{25}\) grams or \(2.4 \times 102^{2}\) kg. The weight of the Earth is about \(6 \times 1024\) kg, so that pile would weigh about .5% of the mass of the Earth, or about a third of the mass of the moon, at \(7.32 \times 10^{22}\) kg. That’s a lot of donuts and a lot of diabetes. Now, atoms are incredibly small, so a mole of water molecules (H\(_2\)O) only weighs about 18g. That’s a manageable mass for a laboratory and for observations, so we will tend to use moles when we talk about experiments.
General Notes:
This is a concept that trips some people up. Always double-check to make sure your conversion is correct. Avogadro’s number is vastly huge. If you divide it instead of multiplying it, you should know. Remember, you will never have a question that will return a ridiculously unrealistic answer.
Fundamental Knowledge and Skills - Mass to Moles Conversions
How to Recognize When You Need to Do It:
If you need to measure out or report the amount of a material, be an element or compound, you need that amount expressed in mass units, typically in units of grams, because you cannot count out individual particles, i.e., atoms or molecules.. Any time the material in question is involved in a chemical formula or in a chemical equation, the amount of that material needs to be expressed as a particle amount, in units of moles.
How to Do It:
The molar mass of a material in units of grams represents one mole of individual particles, be they atoms or molecules. If the amount of a material is given in mass units, typically grams, and the material in question is used in a chemical formula or chemical reaction, this mass amount needs to be expressed in particle units of moles. Consequently, determining the molar mass of the material is the first step. Since the molar mass represents the mass of one mole of particles (atoms or molecules), it is the key to converting a mass amount to a mole amount and a mole amount to a mass amount.
For example, the molar mass of ethanol (C\(_2\)H\(_6\)O) is \(46.07\) g, meaning that \(46.07\) g of ethanol \(=\) \(1\) mole of ethanol. This equation can also be algebraically manipulated and expressed as \(1\) \(=1\) mole of ethanol\(/46.07\) g of ethanol or as \(1 = 46.07\) g of ethanol\(/ 1\) mole of ethanol. If we are given that we have \(23.04\) g of ethanol, and we need that amount expressed in moles, we can multiply this gram amount by the \(1\) mole of ethanol\(/46.07\) g ratio, which has a value of 1, to cancel out the gram units and introduce mole units. Going the other direction, if we are given ethanol as a mole amount, say 2.0 moles, we can multiply this mole amount by the ratio \(46.07\) g \(/1\) mole of ethanol, which also has a value of 1, to cancel out the mole units and introduce gram units.
Why:
Elements are represented in chemical formulas as individual atoms, and atoms, compounds, and molecules react with each other as individual particles. Consequently, material amounts when used in chemical formulas or in chemical reactions must be expressed in particle amounts. However, mass amounts are used when measuring or reporting the amount of a material, because individual atoms or molecules cannot be directly counted as particles; they are far too small and their numbers are far too large.
General Notes:
Converting mass amounts of mole amounts and mole amounts to mass amounts and knowing when you need the amount of a material expressed as a mass or mole amount, is a fundamental skill and one that is the heart of solving the majority of numerical problems in chemistry. You need to know this and understand the whys behind it.
Molecular Mass/Mole Calculation Practice
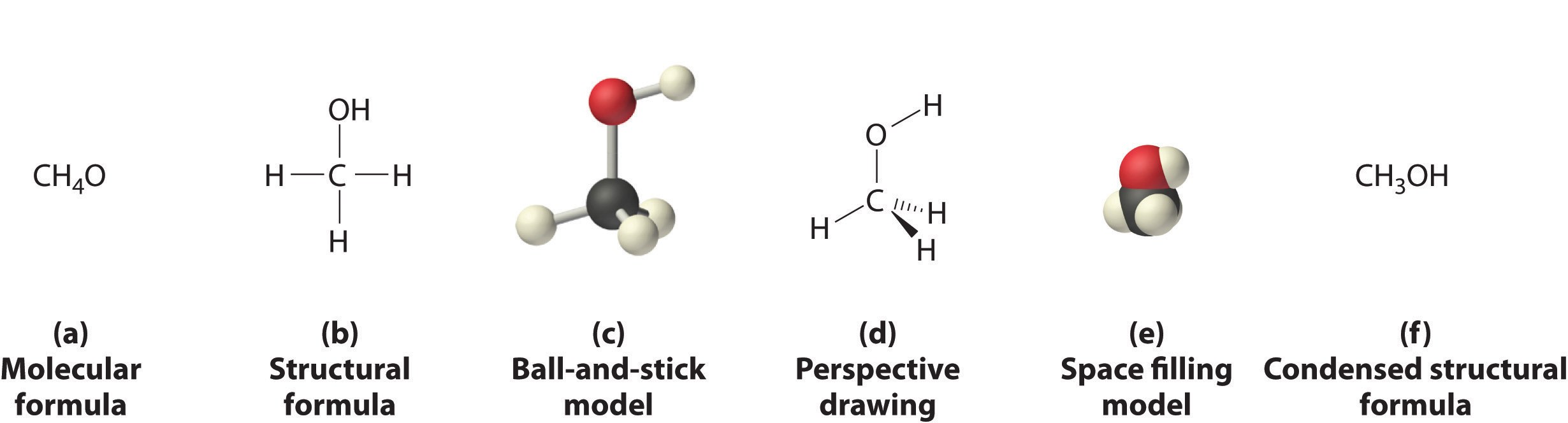
Anonymous, Different Ways of Representing the Structure of a Molecule, LibreTexts (CC BY-NC-SA 4.0).
From a drawing by C. Sentier, executed in Torino at Litografia Doyen in 1856., Avogadro Amedeo, Wikimedia Commons (public domain).
- Unknown , Potassium-2, Wikimedia Commons (CC BY 3.0). [Image has been altered from the original.]
- Marie-Lan Nguyen , Enfant oie Louvre, Wikimedia Commons (public domain).