Chemical Formulas and the Periodic Table of Elements

Elemental Symbols

Dalton published his atomic theory in 1808 and envisioned atoms as hard spheres that combined with other atoms in set ratios. He proposed a set of circular symbols for each element, with the circle representing the fact that atoms were individual particles that joined with each other to form compounds. For example, the compound carbon monoxide was shown as a circle representing a carbon atom joined to a circle representing an oxygen atom. Similarly, the symbol for carbon dioxide was the circle representing a carbon atom joined to two circles representing oxygen atoms.
A few years later, the Swedish chemist Jön Berzelius suggested simplifying the circular elements' symbols and simply using a single letter or a combination of two letters to symbolically represent each element. This is how each of the elements is symbolically represented today. The letter symbol for each element typically uses the first letter of the name of the element, and that may or may not be combined with a second letter. For example, the symbol for the element fluorine is F, and the symbol for the element phosphorus is P, but the symbol for the element lithium is Li, and the symbol for chlorine is Cl. Some exceptions to this system exist because the letters are derived from the Latin name of the element such as, Na for sodium (from the Latin word for sodium or natrium), K for potassium (from the Latin word for potassium or kalium), from the Latin word Fe for iron (from the Latin word for iron or ferum), Cu for copper (from the Latin word for copper or cuprum), and Pb for lead (from the Latin word for lead or plumbnum).
Elemental Symbols Practice
Fundamental Knowledge and Skills - Elements
What You Need to Know:
The names and symbols of the subset of elements are listed on the Exam 1 Study Guide. You should also be able to find them on the periodic table and convert names to symbols and vice versa.
How to Learn It:
In some cases, the element symbol is only the first letter of the element name, but it most often includes a second letter (typically the second letter in the name, but sometimes the last letter). A few elements are exceptions to this general trend. Some of the exceptions to the 'first-two-letter rule' include sodium, potassium, antimony, iron, copper, mercury, tin, silver, gold, and tungsten. This is because their names come from other languages.
It also helps to learn the relative position of these elements of interest on the periodic table and to learn which group they belong to. This will not only help you use the periodic table more efficiently when you need to retrieve information from it, but also help you better associate an elemental name with its symbol.
Why It Matters:
The element names and symbols are a key part of the spoken, written, and symbolic chemical language. Although you will have access to a periodic table, it may or may not have names of the elements along with the symbols. The periodic table you will have on the exam has symbols but no names. Problems may use the name but not the symbol, making it more difficult to access atomic mass information from the periodic table. The bottom line is that you have to have the basic vocabulary to communicate and solve problems.
Chemical and Molecular Formulas
The chemical formula of a compound is a symbolic representation of the compound that not only shows which elements combine to form the compound, but also the relative number of atoms of each element in the compound. The individual elements in a chemical compound are represented by their letter symbols and, if there is more than one atom of the element present in the compound, the letter symbol is followed by a subscripted number to indicate the number of atoms of that element present in the compound. For example, the letter symbol for carbon is C, and the letter symbol for oxygen is O. The compound carbon monoxide consists of one atom of the element carbon and one atom of oxygen. Consequently, carbon monoxide is represented as CO. There are no subscripts because there is only one atom of carbon and one atom of oxygen in the compound. Carbon dioxide, however, is represented as CO\(_2\) because it consists of one atom of carbon and two atoms of oxygen. Baking soda has 1 atom of the element sodium (symbol Na), one atom of the element hydrogen or H, one atom of the element carbon, and 3 atoms of the element oxygen, and is represented as NaHCO\(_3\). The chemical formulas of carbon monoxide, carbon dioxide, and baking soda all represent the elemental and atomic composition of each of these compounds. These symbolic representations are known as chemical formulas.
The compounds, carbon monoxide and carbon dioxide, are composed of individual particles. Every particle of carbon monoxide is composed of one atom of carbon and one atom of oxygen, and each particle of carbon dioxide is composed of one carbon atom and two oxygen atoms. Compounds like these are called molecular compounds, and the individual particles of these compounds are referred to as molecules. The chemical formulas of molecular compounds like carbon monoxide and carbon dioxide are more specifically referred to as molecular formulas.
Much of learning chemistry, as in life, is about establishing and defining relationships. The relationship between an element, a compound, and a molecule is a good example. You can define each one, but it is better to ask how these terms are related to each other; how are they similar, and how do they differ? For example:
All substances are derived from a common set of elemental building blocks. A molecule is a unit or particle that contains more than one atom. H2 and O2 are molecules because they contain more than one atom bound together as a unit or particle. However, they are also classified as elements because each molecule only contains one type of element in the molecular unit. H\(_2\)O is a molecule because it contains more than one atom tied together as a unit or particle, but it is also a compound because there is more than one type of element present in the molecule.
Molecular and Chemical Formulas Transcript
Fundamental Knowledge and Skills - Chemical Formulas
What You Need to Know:
How to write a chemical formula.
How to Learn It:
Chemical formulas are simple: they tell you how many atoms of each type of element are present in the compound or molecule.
A subscript following an elemental symbol indicates the number of atoms of that element in the compound or molecule. The lack of a subscript indicates that there is only one atom of that element in the molecule. For example, H\(_2\)O tells us two things: there are two hydrogen atoms and one oxygen atom in each water molecule.
The molecular formula C\(_8\)H\(_9\)NO\(_2\) indicates that there are 8 carbon atoms, 9 hydrogen atoms, 1 nitrogen atom, and 2 oxygen atoms present in a single molecule of this compound. If the molecule consists of 3 carbon atoms, 1 oxygen atom, and 8 hydrogen atoms, its molecular formula is written as C\(_3\)H\(_8\)O.
Remember, if you change a subscript in a molecular formula, you change the molecule itself.
Why It Matters:
Chemists use molecular formulas to communicate the atomic composition of molecules, and chemical structures to communicate the way that atoms are bound to each other. It is important to learn how to read and use molecular formulas.
The Early Periodic Table of the Elements
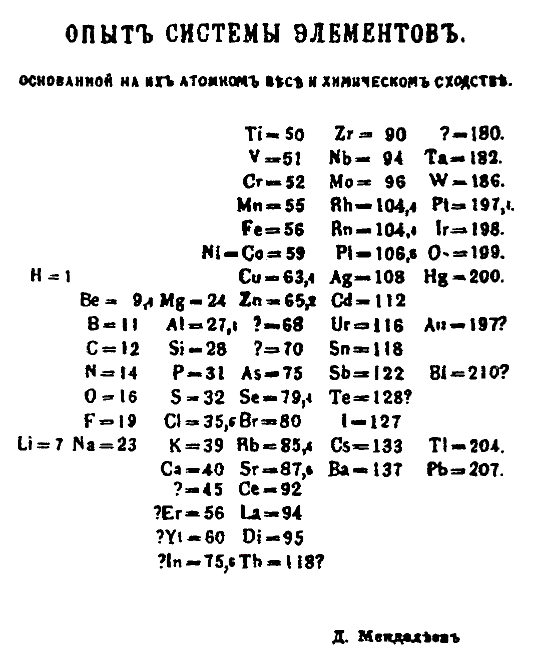
Throughout the nineteenth century, the most characteristic and defining property of an element was its unique atomic mass. There were several attempts to find an underlying pattern or order that connected all of the elements. Although many individuals tried to find some organizing pattern that connected the chemical properties of the elements with their atomic mass values, the most successful of these was the Russian chemist Dmitri Mendeleev. In 1869, as Mendeleev was writing a general chemistry textbook, he discovered that if the elements were regularly organized in columns and rows, certain elements were grouped in a regular, recurring, or “periodic” pattern.
Although Mendeleev’s arrangement of the elements was basically in order of increasing elemental atomic mass values, he made some notable exceptions. Because he recognized that the chemistry of I matches that of the F, Cl, and Br group and the chemistry of Te matches that of the neighboring O, S group, he swapped the positions of the elements iodine (I) and tellurium (Te), despite the fact that the atomic mass of I is less than that of Te. He reasoned that placing elements that have similar chemistries together was more important than ordering them by increasing atomic elemental mass. However, to keep some elements together, he was forced to leave blank spots in his ordered elemental arrangement and predicted that these blank spots would be filled by elements yet to be discovered. He was even bold enough to predict the expected atomic mass values of these undiscovered elements as well as their physical and chemical properties. He published his first periodic table of the elements in 1869, and within 16 years, three of the elements he predicted were discovered. These were the elements scandium, germanium, and gallium.

Periodic Table 1: Mendeleev Transcript
Fundamental Knowledge and Skills - Mendeleev and the Periodic Table of the Elements
What You Need to Know:
You need to understand the reasoning behind the way the periodic table is organized, and why there are exceptions to the general trend of ordering elements by increasing atomic mass. You’ll also need to know the general story of Mendeleev creating the basis of the modern periodic table, and you should also be able to explain why Mendeleev left gaps in his original periodic table.
How to Learn It:
Mendeleev organized the periodic table similarly to the card game, solitaire. Imagine a solitaire game with 4 columns that correspond to suits: diamond, heart, spade, and club. Then the cards proceed in descending order from king to ace. In other words, the numbers correspond to rows: the first row corresponds to kings, the second row corresponds to queens, etc. If you wanted to find the nine of spades, you would simply look at the spades column, then go down until you found the row with the nines.
The modern periodic table of elements has a similar organizational method. The columns are organized into ‘periods’ or ‘families’- groups of elements with similar properties. The rows are organized by increasing atomic mass, except for the rare exceptions where organizing by increasing mass and similar properties have a conflict. In these rare exceptions, the elements are arranged in columns with similar properties. One of these exceptions is Cobalt and Nickel. Cobalt has an atomic mass of 58.933 atomic mass units, while Nickel has an atomic mass of 58.693 amu. However, Nickel is to the right of Cobalt, despite having more mass. This is because Nickel has similar properties to Platinum, and Cobalt has similar properties to Rhodium and Iridium. Chemical similarities take precedence over mass in Mendeleev’s periodic table.
You may notice that the atomic numbers increase across the rows in the periodic table, indicating that with increasing numbers of protons often comes increasing mass. The existence of protons and the techniques to quantify the number of protons in an element had not yet been discovered, but Mendeleev’s organization resulted in the perfect ordering of the elements by atomic number.
Reactivity is largely determined by the number of valence electrons orbiting the nucleus of an atom. The periodic table is organized so that we know how many valence electrons there are for each atom, and every column contains the same number of valence electrons, which explains the remarkably similar reactivities that elements in the same column have.
Mendeleev left gaps in his periodic table where some elements had not been discovered yet. He was able to correctly predict the existence of these elements, their approximate masses, and their reactivities. For example, he left two spaces in between Zinc and Arsenic, and within 15 years, both Gallium and Germanium were discovered. His predicted atomic masses, densities, and reactivities were very similar to the actual characteristics of Gallium and Germanium. He was able to predict the masses by averaging the masses of the neighboring elements bordering the empty space to each side. He was able to predict their reactivities by looking at the column (or “family”) that the unknown element belonged to, and reasoning that they likely reacted similarly.
Why It Matters:
The periodic table is the best tool you have in your chemistry toolkit. There is a lot of information encoded in the organization of the modern periodic table, which has been revised and updated several times since Mendeleev first organized it. Your periodic table will become your best friend in chemistry. You can learn about an element’s reactivity with just a quick glance at the periodic table. Later, when we discuss periodic trends, we will discuss how you can predict atomic size, the amount of energy it takes to pull an electron off an atom, how much energy is released when an atom gains an electron, how much ‘pull’ a given atom’s nucleus has on surrounding electrons, and more. Use this tool, and learn to love it.
The Modern Periodic Table of the Elements
The modern periodic table of the elements parallels Mendeleev’s early periodic table. It displays the one or two-letter symbol for each element along with its unique atomic mass in numerical order, beginning with hydrogen as number one. Elements with similar chemical properties are found grouped together in vertical columns. Some of these groups or families of elements have names that are commonly used to identify the group. For example, the far left-hand column or Li, Na, K, Rb, Cs, and Fr, is referred to as the alkali metals. Although the element H is often found at the top of this column, its chemistry is not the same as the elements Li through Fr and is not considered part of the alkali metals group. The column of elements directly to the right of the alkali metals is referred to as the alkaline earth metals. This group includes the elements Be, Mg, Ca, Sr, Ba, and Ra. On the other side of the table, the far right-hand column, or He, Ne, Ar, Kr, Xe, and Rn, form the group known as the noble gases. The column immediately to the left of the noble gases, or F, Cl, Br, I, and At, comprises the group known as the halogens, from the Latin, meaning salt formers. These four groups, alkali metals, alkaline earths, noble gases, and halogens, are commonly referred to by their group name.
The individual rows on the periodic table are called periods and are identified by number, with the first period consisting of the row containing just the elements H and He. The second period is the row commencing on the left-hand side of the table with Li and proceeding through Ne. Likewise, the third period begins with the element Na; the fourth period with the element K, and so forth. In addition to classifying the elements on the periodic table by rows and columns, some blocks of elements are grouped together. The two far left-hand columns and the six columns on the far left-hand side of the table are referred to as the main group elements. The ten columns separating the two blocks of the main group elements are referred to as the transition metals. The top row of fourteen elements of the two separate rows at the bottom of the table is referred to as the lanthanides or rare earth elements, whereas the bottom row of fourteen elements comprises the actinides.
A typical periodic table of the elements lists the symbols and atomic number, and average atomic mass of the elements. Mendeleev recognized that elements that behaved similarly to each other belonged together. For example, the elements F, Cl, Br, and I all form similar compounds with the element H: HF, HCl, HBr, and HI. When he arranged the elements by their ever-increasing atomic mass values, with a few exceptions, he saw a pattern of rows and columns that placed these groups of similar elements together. He also realized that he had to put in blank placeholders for elements yet to be discovered to complete the pattern. Suggestion: When you are learning the elements and their symbols, locate those elements on the periodic table and identify the group or classification that they belong to. For example, when learning chlorine (Cl), find chlorine on the far right-hand side of the periodic table and one column to the left, which places it in the halogen group. Similarly, locate sodium in the far left-hand column in the alkali metal group. Both of these elements are also classified as main group elements.
Modern Periodic Table Transcript

For an accessible version of the Periodic Table, go here.
Sylwia Schreck/stock.adobe.com, Naturelemente , Adobe Stock (Adobe Stock Education License).
Dalton, Dalton's symbols of the elements, Wikimedia Commons (CC BY 4.0).
atwailee, Water droplet , FreeImages (Used with permission).
penlix, Image gratuite de Piscine tranquille à Hammamet, Tunisie à télécharger, FreeImage
Enricoros, SiliconCroda, Wikimedia Commons (public domain). [Image has been altered from the original.]
anna076, Imagen de stock gratuita de Pieza decorativa de sarmiento de hierro forjado para descargar, FreeImage
michaelaw, Copper bundt pan in kitchen, FreeImages (Used with permission).
Hi-Res Images of Chemical Elements, Silverchain, Wikimedia Commons (CC BY 3.0).
Unknown, Kinkaku3411, Wikimedia Commons (public domain).
grafbea, Ornate silver ring with red gemstone, FreeImages (Used with permission).
sooperkuh, Aluminum extrusion with vertical grooves, FreeImages (Used with permission).
iprole, Imagen de stock gratuita de Jarra de vidrio clara sobre fondo gris para descargar, FreeImages (Used with permission).
Marzie, Free Crumpled ziplock bag on turquoise background stock image to download, FreeImage
Unknown, photo of Dmitry Ivanovich Mendeleev, Wikimedia Commons (public domain).
Dimitri Mendeleev, Facsimile of Mendeleev's 1869 periodic table of the elements., 1869, Wikimedia Commons (public domain).
Е.Л. Мрозовская, Mendeleev Photographische Gesellschaft, Wikimedia Commons (public domain).
en:user:foobar, Gallium crystals, Wikimedia Commons (CC BY-SA 3.0).
Gibe , Germanium, Wikimedia Commons (CC BY-SA 3.0).
Mondalor, Lithium hydroxide, Wikimedia Commons (CC BY-SA 3.0).
Jurii, Helium-glow, Wikimedia Commons (CC BY 3.0).
Jurii, Argon-glow, Wikimedia Commons (CC BY 3.0).
Jurii, Krypton-glow, Wikimedia Commons (CC BY 3.0).
Jurii, Xenon-glow, Wikimedia Commons (CC BY 3.0).
socyo, Table salt against a red background , FreeImages (Used with permission).
NOAA, Magic Mountain metallic sulfide ore, Wikimedia Commons (public domain).