The Basic Structure of the Atom
Electricity Basics and Coulomb's Law
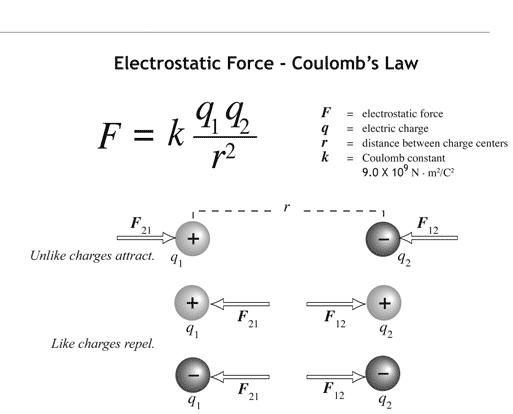
A sustained source of electricity was not available until the Italian scientist Alessandro Volta invented the first electric battery in 1800. The elements potassium, sodium, and calcium were discovered shortly thereafter by treating various materials with electricity from the battery. This hinted at the possibility that matter, on some level, had an electrical nature. Before the discovery of the battery, “static electricity” was classified as carrying either a positive or negative charge. Like charges, either positive and positive or negative and negative repel each other and opposite charges, positive and negative, attract each other. Furthermore, it was known that the greater the charge on the objects, the stronger the repulsive or attractive interaction between them. It was also known that objects carrying the same amount of charge were more strongly attracted to or repelled by each other the closer they were to each other. Taken together, the strength of the electrical force of attraction between opposite charges or the repulsion between like charges increases as the amount of charge increases and decreases as the distance between the charges increases. The French scientist Charles Augustin de Coulomb expressed these observations in mathematical form as \(F \propto \frac{q_1 q_2}{d^2}\). This relationship, known as Coulomb’s law, states that the electrical force (\(F\)) is directly proportional to the amount of charge on object 1 (\(q_1\)) and on object 2 (\(q_2\)) and decreases dramatically as the distance (\(d\)) between the two charges increases.
Coulomb’s Law – Charge and Distance

Electrostatic Properties Transcript
Fundamental Knowledge and Skills - Coulomb’s Law
What you need to know:
Coulomb’s law states that the attractive or repulsive electrical forces experienced by two charges are directly proportional to their charges and inversely proportional to the distance between the two charges. You should know the equation associated with Coulomb’s law, where \(q_1\) is the charge that the first particle has, \(q_2\) is the charge that the second particle has, and \(d\) is the distance between the particles.
\(F \propto \frac{q_1 \times q_2}{d^2}\)
In other words, charge and distance determine the force that two charges exert on each other.
How to learn it:
Coulomb’s law applies when there are two or more charged particles. Particles that are the same charge repel each other. Particles with opposite charges attract each other. Within atoms and molecules, protons repel protons and electrons repel electrons. Protons are attracted to electrons, and vice versa. Neutrons, which have no charge, are not electrostatically attracted or repelled by protons or electrons.
Coulomb’s Law tells us the magnitude of the force of attraction or repulsion experienced by charged particles.
\(F \propto \frac{q_1 \times q_2}{d^2}\)
This is the equation that you must become familiar with. Although, in this course, we, will not calculate the actual force, the relationship between charge, distance, and force is vital for your understanding of chemistry.
This equation suggests that if both particles are positive or both are negative, the product will be positive, and thus the force will be positive. Thus, a positive force is defined as a repulsive force. If one particle is positive and the other is negative, the product will be negative. Thus, a negative force is defined as an attractive force.
There are two ways to increase the force experienced between two particles. You can either increase the charges of the particles or you can decrease the distance between them. In the same way, there are two ways to decrease the force experienced between two particles. You can either decrease the charges of the particles or you can increase the distance between them.
The electrical force is similar to the force between two magnets. If you put two North or South ends of a magnet together, they repel. If you put the North end of a bar magnet close to the South end of another bar magnet, they attract. The closer you put the North and South ends of the magnets together, the greater the attractive force. They will ‘snap’ together when you get too close, and it takes a lot of force to pry the magnets apart. If you were to put the North end of a larger, industrial magnet close to the South end of a regular bar magnet, there is an incredibly strong attractive force, and they will ‘snap’ together.
Why it matters:
The Electrical Nature of the Atom and Basic Structure of the Atom
In 1897, the British scientist J.J. Thomson showed that all matter was composed of negatively charged particles very much smaller than the smallest atom. He realized that these negatively charged particles were a fundamental part of all atoms. Although he was not able to accurately determine the mass of these particles, it was clear that their mass was far less than an atom of the lightest known element, hydrogen. These negatively charged subatomic particles, which are far smaller than an atom, are what we know today as electrons.
Atoms are electrically neutral, meaning that the total positive and the total negative charge within the atom are the same. In light of his discovery of the negatively charged electron, Thomson proposed a model of the atom as a hard sphere, as envisioned by Dalton, with the individual electrons embedded within positively charged material. This became known as the plum pudding, or blueberry muffin, model of the atom. The electrons were seen as the raisins in the plum pudding, or the blueberries in the muffin, surrounded by a positively charged cake-like material.
A few years later, Ernest Rutherford proposed a radically different view of the atom. After careful analysis of how positively charged alpha rays were deflected by the atoms in a thin gold foil, he announced that all of the positive charge, and essentially all of the mass of the atom, was found in a vanishingly small volume in the center of the atom's subatomic region, which he referred to as the nucleus of the atom. In the following years, he was able to prove the existence of a second sub-atomic particle located in the nucleus that carried a positive charge. He named this particle the proton.
A single proton has a positive charge equal but opposite to that of a single electron and a mass about the same as a single hydrogen atom. In contrast, a single electron has a mass that is about \(\frac{1}{2000}\) of a proton or hydrogen atom. The massive proton is found in the extremely small nucleus at the center of the atom, while the far less massive electron can be found in the very large non-nuclear volume of the atom. The third principal subatomic particle, the neutron, was not discovered until 1932. Like the proton, the neutron is confined to the atomic nucleus and has a mass that is very close to that of a proton, but it carries no charge. It is electrically neutral, hence the name neutron. Because the mass of an electron is so small compared to the mass of a proton or neutron, the mass associated with a single atom is almost entirely due to the number of protons and neutrons in its nucleus.
Electrical nature of the atom:
- Thompsons' cathode ray experiment revealed the negatively charged electron as a fundamental building block of matter and resulted in the revised “plum pudding or blueberry muffin” model for the atom.
- Rutherford’s gold foil experiment led to the understanding that all of the positive charge (protons) and essentially all of the mass of the atom (protons plus neutrons) is concentrated in the extremely small volume of the atomic nucleus and the nuclear model of the atom.
Thomson's Cathode Rays Transcript
Thomson's Plum Pudding Model Transcript
Rutherford Gold Foil Experiment Transcript
Subatomic Particles Transcript
Atomic Number, Isotopes, and Mass Number

- You need to be able to determine the number of protons, neutrons, and electrons in a given isotope of an element. The number of protons corresponds to an element's atomic number (Z), the atomic weight or mass of an element minus its atomic number equals the number of neutrons; the atomic number ( number of protons) equals the number of electrons in a non-charged neutral atom.
- You need to be able to calculate the average atomic mass of an element given is relative isotopic mixture. If you assume that you have 100 atoms, then the number of atoms of each type of isotope matches the percent. The average atomic mass is obtained, if the number of atoms of each type of isotope times its relative mass for all of the isotopes are added together and divide by 100.
The number of protons found in the nucleus of an atom, known as the atomic number. The atomic number not only determines the total positive charge within the nucleus of an atom, but it is also the unique defining characteristic of each element. Furthermore, it correlates with an element’s position on the periodic table. Each element has a unique number of protons and all atoms of the same type of element have identical numbers of protons in their nuclei. However, the number of neutrons in the nuclei can vary. For example, all carbon atoms, atomic number 6, have six protons in their nuclei, but while most carbon atoms have six neutrons in their nuclei, about 1% of carbon atoms have seven neutrons. Since the mass of a proton and a neutron are essentially the same, those atoms with seven instead of six neutrons are more massive.
On the atomic scale, it is convenient to express mass in units of atomic mass units (amu) because one amu is almost exactly the mass of one proton or one neutron, which in turn, is almost identical to the mass of a single hydrogen atom. Expressed in amu, the mass of the carbon atom with six protons and six neutrons is 12, and the mass of the carbon atom with six protons and seven neutrons is 13. Because these atoms are both atoms of carbon and have the same number of protons, they occupy the same place on the periodic table. However, they differ in mass because they differ in the number of neutrons. These two carbon atoms are referred to as isotopes, from the Greek words iso meaning “equal” or “same” and topos meaning “place.” Isotopes are atoms of the same element, meaning they have the same atomic number (number of protons) and consequently the same position on the periodic table but differ in mass because they differ in the number of neutrons. Isotopes are distinguished from each other by their mass number, which is the sum of both the protons and the neutrons in the atomic nucleus. When required, the mass number is written to the left of the symbol and superscripted, preceding the elemental symbol (e.g., \({}^{12}\mathrm{C}\) or \({}^{13}\mathrm{C}\)).

Elemental Notation and Isotopes
Atomic Number, Isotopes, and Mass Number Practice
Fundamental Knowledge and Skills - Atomic Number, Isotopes, and Mass Number
What you need to know:
You need to know how to determine the identity of an element from its atomic number. You also need to know how to determine the number of neutrons present in an element given its atomic number and mass number. In addition, you need to understand the difference between isotopes of a particular element and how to read the periodic table to know the number of protons and neutrons present in an atom.
The atomic number is the number of protons present in an atom. The mass number is the total number of protons and neutrons present in the nucleus of an atom.
How to learn it:
The identity of an element is dependent on how many protons are present in the nucleus. Change the number of protons in the nucleus and you change what element you have. The number of neutrons present in the nucleus is variable. Neutrons exist mainly to keep the mass of the nucleus large enough to be stable. If the nucleus was composed solely of protons, then the proximity of the positive charges would result in the repulsive forces of the protons overcoming the strong nuclear force keeping them together. Neutrons exist to add additional strong nuclear forces so that the nucleus could be stable.
Because the number of neutrons necessary to keep the nucleus stable for a particular element is relatively variable, atoms of the same element may have different numbers of neutrons. Isotopes are atoms of the same element that have the same number of protons, but different numbers of neutrons. Thus, isotopes are elements that have the same atomic number, \( Z \), and different mass numbers, \( A \).Let’s look at a real example. Uranium has several isotopes. Uranium-235 is the isotope of Uranium that is used in atomic fission reactors and atomic bombs. Uranium-238, however, is the most common uranium isotope. Both isotopes of Uranium contain \( 92 \) protons. \({}^{235}\mathrm{U}\), then contains \( 92 \) protons and \( 143 \) neutrons. \({}^{238}\mathrm{U}\) contains \( 92 \) protons and \( 146 \) neutrons.
As you study introductory chemistry, your periodic table is your best friend.
You can determine atomic and mass numbers from looking at the periodic table.
It should be noted that the atomic mass on the periodic table is an average atomic mass, calculated from the relative abundancies of all isotopes of that element.
Why it matters:
It’s important to understand that the identity of an element is dependent on its number of protons, and its mass is dependent on its number of protons and neutrons. Isotopes are especially important for nuclear chemistry and nuclear reactors.
Average Atomic Mass
 The atomic mass values printed on a typical periodic table of the elements represent the average mass value of the naturally occurring mixture of isotopes. For example, 98.9%, or about 99 in every 100, carbon atoms in nature have 12 protons and 12 neutrons with a mass of \(12 \, \mathrm{amu}\), but 1.1%, about 1 in every 100, carbon atoms have 12 protons and 13 neutrons with a mass of \(13 \, \mathrm{amu}\). Consequently, the mass of carbon is 12.011 on the periodic table, slightly above 12.00 amu because of the small amount of the heavier isotope, \({}^{13}\mathrm{C}\). Another example is the element chlorine which has 17 protons. In nature, 74% of the chlorine atoms are \({}^{35}\mathrm{Cl}\) with 18 neutrons, and 24% are \({}^{37}\mathrm{Cl}\) with 20 neutrons. The atomic mass of Cl is 35.45 amu and reflects this three to one isotopic ratio.
The atomic mass values printed on a typical periodic table of the elements represent the average mass value of the naturally occurring mixture of isotopes. For example, 98.9%, or about 99 in every 100, carbon atoms in nature have 12 protons and 12 neutrons with a mass of \(12 \, \mathrm{amu}\), but 1.1%, about 1 in every 100, carbon atoms have 12 protons and 13 neutrons with a mass of \(13 \, \mathrm{amu}\). Consequently, the mass of carbon is 12.011 on the periodic table, slightly above 12.00 amu because of the small amount of the heavier isotope, \({}^{13}\mathrm{C}\). Another example is the element chlorine which has 17 protons. In nature, 74% of the chlorine atoms are \({}^{35}\mathrm{Cl}\) with 18 neutrons, and 24% are \({}^{37}\mathrm{Cl}\) with 20 neutrons. The atomic mass of Cl is 35.45 amu and reflects this three to one isotopic ratio.Average Atomic Mass Transcfipt
Fundamental Knowledge and Skills - Average Atomic Mass
How to recognize when you need to do it:
Typically, you are given the natural abundances of isotopes of an element and are asked to determine the average atomic mass. Here’s an example, “Carbon-13 is found with a natural abundance of 1.1%, with the remainder being Carbon-12. Carbon-13 has a mass (in amu) of 13.00335 and Carbon-12 has a mass of 12.0000 amu. What is the average atomic mass of Carbon?”
How to do it:
Many elements have several stable isotopes. For example, Carbon has 6 protons, and always does. However, it can have either 6 or 7 neutrons. The 6-neutron species of carbon is known as Carbon-12 or \({}^{12}\text{C}\) and is the most common form of carbon. Only 1.1% of all carbon is the 7-neutron species, known as Carbon-13 or \({}^{13}\text{C}\). Carbon-14, which is important for carbon dating, is only present as one part per trillion, so we will leave it out of our approximation for now.
Carbon-12 and Carbon-13 react exactly the same. Thus, any sample of carbon you pick up should have roughly the same proportions of \({}^{12}\text{C}\) and \({}^{13}\text{C}\). If you have a block of graphite (carbon) and wish to know how many atoms of carbon there are, you must use the molar mass of carbon. However, which molar mass should we use? The molar mass of \({}^{12}\text{C}\) is 12.000 grams/mol of Carbon atoms, whereas the molar mass of \({}^{13}\text{C}\) is 13.00335 grams/mol of Carbon atoms. In order to precisely determine the number of carbon atoms present in our graphite sample, we must determine the average atomic mass of carbon, which is a mix of both isotopes.
Quick side note: The mass of an atom is often represented with amu, or atomic mass units. 1 amu is defined as \(\frac{1}{12}\) of the mass of a carbon atom and is roughly similar to the mass of a proton or neutron. 1 amu is equivalent to \(1.66054 \times 10^{-27}\) kg. We will use amu when we talk about the masses of individual atoms. We use grams/mol when we talk about molar quantities of atoms.
In an average sample of carbon atoms, 1.1% are \({}^{13}\text{C}\), while 98.9% are \({}^{12}\text{C}\). To determine the weight due to each component, we must multiply the mass of the isotope by the percentage (expressed in decimal form).
Mass due to \({}^{13}\text{C}\) = 0.011 × 13.00335 amu = 0.14303685 amu
Mass due to \({}^{12}\text{C}\) = 0.989 × 12.00000 amu = 11.868 amu
Weighted Average Total Mass = 0.14303685 + 11.868 = 12.01103685 amu
Thus, the weighted average atomic mass is 12.011 amu or 12.011 g/mol. If we want to know the number of moles of carbon atoms in a sample of graphite, we will use the weighted average atomic molar mass. For example, if there were 100 grams of graphite, there would be \(100 \text{ grams C} \times \frac{1 \text{ mol}}{12.011 \text{ grams C}} = 8.3257 \text{ moles of carbon atoms}\)
Why It matters:
Naturally occurring elements are mixtures of different isotopes, each with a unique atomic mass. The atomic mass values shown on the periodic table reflect this. Knowing what this mix is helps us to understand why atomic mass values are not whole-number ratios of each other. However, there are cases where specific isotopes are used and not the naturally occurring mix. We use isotopes to determine the age of certain objects with carbon-dating, which uses the proportions of \(\left[^{14}\text{C}\right]\) present in organic objects. Plants take in carbon-containing \(\text{CO}_2\) and use that carbon to form molecular structures; thus, plant-based papyrus can be carbon-dated. Older materials have less \(\left[^{14}\text{C}\right]\).
Unknown, Coulomb's law, Physics.stackexchange
Unknown, Water , Uknown
Esmaeil Mehdizadeh, Kun Chen, Amir Golabzaei, Figure 1. Electrostatic force between electric charges, Taylor & Francis Online (CC BY 4.0).
Unknown, J.J Thomson, Wikipedia (public domain).
Unknown, Hans geiger, Wikipedia (public domain).
Unknown, Ernest Marsden , National Library
Sadi Carnot, Ernest Rutherford2, Wikipedia (CC BY-SA 3.0).
Unknown, Ernest Marsden , National Library
golfingles, golf ball on green grass, Flickr
xsxecutor, Imagen de stock gratuita de Vista panorámica de un paisaje de campo de golf para descargar, FreeImages
Encyclopædia Britannica, James Chadwick , Encyclopedia Britannica
Unknown, An element is represented by symbols like that shown below., Theory
BruceBlaus, Hydrogen Isotopes, Wikimedia Commons (CC BY 3.0).
Uknown, Swan Couple, Uknown
pontuse , Free Pushpin on Vintage New York Map stock image to download , freeimages.com (public domain).
Unknown, He- element, Mrphysics