Matter Consists of Individual Particles or Atoms
Elemental Composition
The amount of each element present in a homogeneous, pure material (meaning a material that contains only one type of element or compound) is directly related to the mass of the material. This is in contrast to a mixture that is heterogeneous and contains more than one type of element or compound. Identical pure samples of the same material contain the same elements in the same relative mass amounts. For example, an \(18\) g sample of pure water contains \(16\) g of the element oxygen and \(2\) g of the element hydrogen. This \(16\) g to \(2\) g mass ratio of oxygen to hydrogen is present in any sample of pure water, meaning that a \(36\) g sample of water would have \(32\) g of oxygen and \(4\) g of hydrogen. There are always \(16\) g of oxygen for every \(2\) g of hydrogen, or, if these numbers are divided by \(2\), \(8\) g of oxygen for every \(1\) g of hydrogen. This \(8\) to \(1\) oxygen to hydrogen mass ratio defines the compound water, and is referred to as the elemental composition of water. Every pure material has its own defining elemental composition, which is characteristic of that material and functions as the elemental recipe for a material.


Elements and Compounds Transcript
Law of Definite Proportions Transcript
Law of Definite Proportions Practice
Fundamental Knowledge and Skill—Atoms, Molecules, Elements, and Compounds
What You Need to Know:
There is a difference between an atom, a molecule, an element, and a compound.
How to Learn It:
Atoms are the basic unit or building block of both chemical elements and compounds. An element is comprised of a collection of a specific kind or type of atom. For example, pure gold is an element, as it is made up of a collection of only gold atoms. Compounds are substances that consist of several types of elements chemically bound together. Glass is a compound made up of many atoms of silicon and oxygen bound together. Molecules are collections of atoms that are chemically bound together, forming a distinct structural unit. Again, for example, if atoms were Legos, then a house made of Legos would be a molecule. A house made wholly out of red bricks would be a molecule and consist of only one element. A house made out of red and blue bricks would be a molecule and a compound. A single red brick would be both an element and a single atom in this analogy.
Why It matters:
Correct terminology is important, especially as you continue to learn chemistry. Speaking "chem-ese" fluently will help your understanding of chemistry and increase your ability to communicate with other scientists.
Fundamental Knowledge and Skills—Law of Definite Proportions
What You Need to Know:
A pure compound always has the same elemental composition that can be expressed as a specific elemental mass or atom ratio.
How to Learn It:
All pure materials have an elemental recipe, meaning that the elements forming that compound combine in the same mass or atom ratios. The specific elements and the amount of each present in the material actually define the material. If you change the elements or the amount of each element in the material, you will get a different material, as you have changed the recipe. This means that pure water in Norway and pure water in Michigan have the same composition, 11.2% by mass hydrogen and 88.8% by mass oxygen. If you were to find the atomic ratio of hydrogen to oxygen, you would find it is \(2:1\). We denote this atomic ratio using chemical formulas, like H\(_2\)O. The subscript following H shows that there are two hydrogen atoms for every one oxygen atom.
Think of dihydrogen monoxide, a compound that contains two hydrogen atoms for every one oxygen atom. In a gallon of pure dihydrogen monoxide, there are roughly \(1.27 \times 10^{26}\) molecules of H\(_2\)O. Every one of these molecules has the same composition and therefore the same properties. Dihydrogen monoxide, or water, is essential for human life. If you were to somehow cause this molecule to gain an oxygen atom, you would form hydrogen peroxide or H\(_2\)O\(_2\), which is a toxin that oxidizes and damages molecules in your body. By changing the ‘recipe’, you have changed the properties of the molecule itself and you have formed a new molecule.
Why It Matters:
The law of definite proportions was an observation by Joseph Proust, a French scientist. However, scientists noted that sometimes several different compounds were made when you mix two or more elements. This led to the discovery of the law of multiple proportions, which was key to the widespread acceptance of the atomic theory raised by John Dalton.
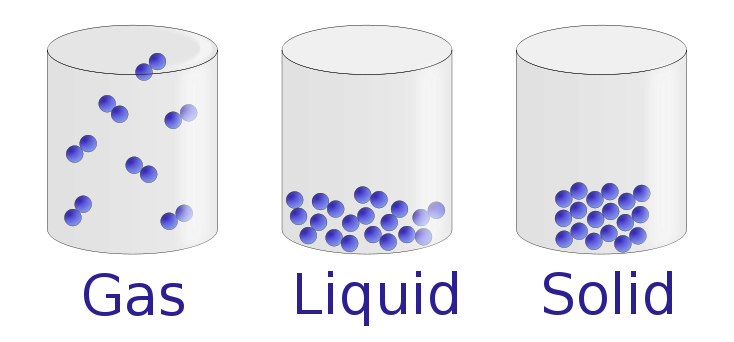
Yelod, States of matter, Wikimedia Commons (CC BY-SA 3.0).
Lukasz Machowczyk/stock.adobe.com, Single drops of light blue and red ink falling down into the glass full of water in the studio. Reflecting surface and gradient background, (Adobe Stock Education License).
365psd.com, Free Vector Water Wave Background Free Vector Download, FreeImage (Used with permission). [Image has been altered from the original.]
Unkown, Waves , Unknown
Jurii, Hydrogenglow, Wikimedia Commons (CC BY 3.0).
somadjinn, Clouds against a vibrant blue sky, Unknown (Used with permission).
Unkown, Ocean waves , Unknown
Mafi48, Chlor 1a, Wikimedia Commons (public domain).
Dnn87, Na (Sodium), Wikimedia Commons (CC BY-SA 3.0).
inya, Free Close-up of white sugar cubes stock image to download, FreeImage
Mario Sarto, Brillanten, Wikimedia Commons (CC BY-SA 3.0).
wax115, Glass of milk, FreeImages (Used with permission).
Unkown, Pile of flour on white table, Unknown
Unknown, Portrait of Joseph Louis Proust, Wikimedia Commons (public domain).